Nuclear Equations
12PHY - Atomic and Nuclear Physics
Finn Le Sueur
2024
Akoranga 6 Te Whāinga Ako
- Balance nuclear equations using the knowledge of the conservation of atomic and mass numbers.
Write the date and te whāinga ako in your books
Alpha Decay
Complete this cloze
In alpha emission, the decaying atom loses 2 protons and 2 neutrons.
This means that the atomic number of the decaying atom is ________ by ________ and the mass number is ________ by ________.
Pātai Tahi: Fill out this table for \(\alpha\) decay
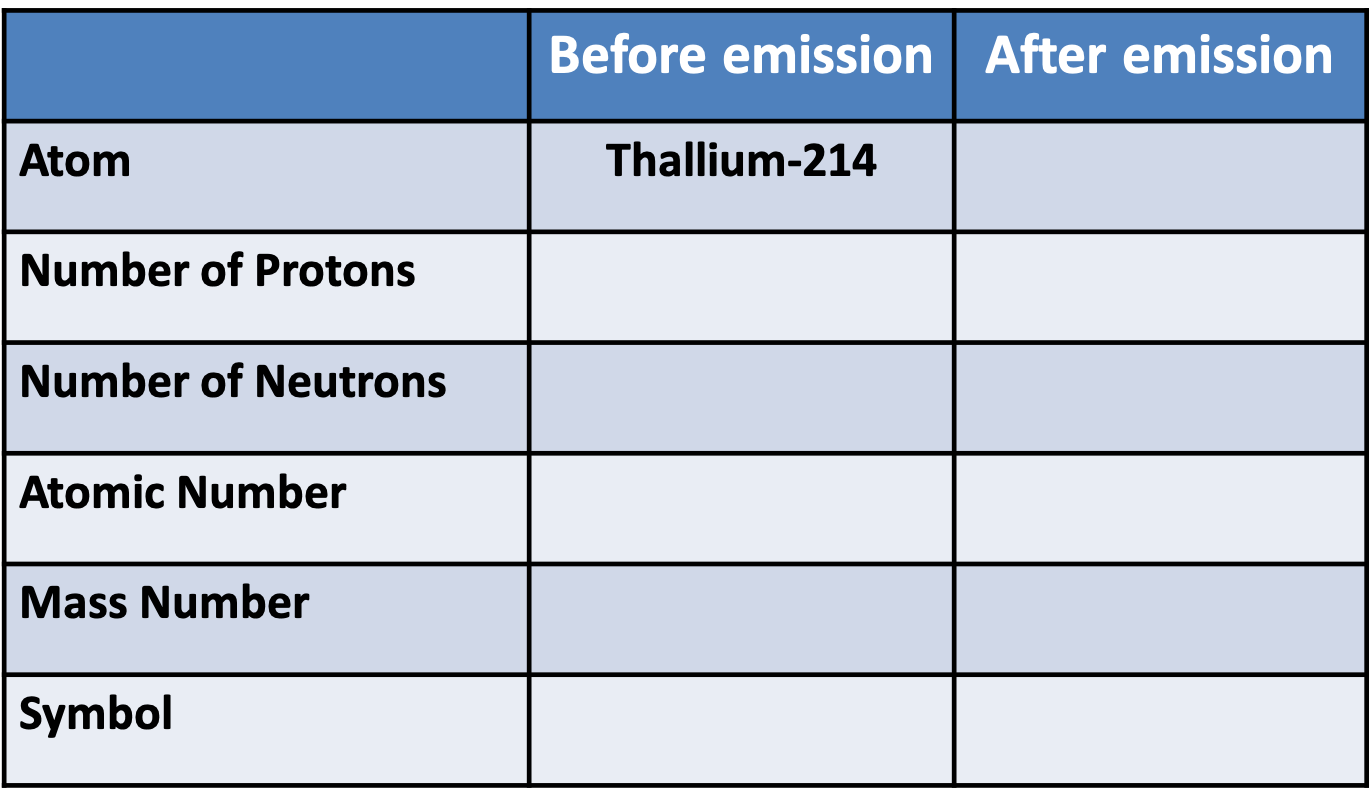
Whakatika Tahi
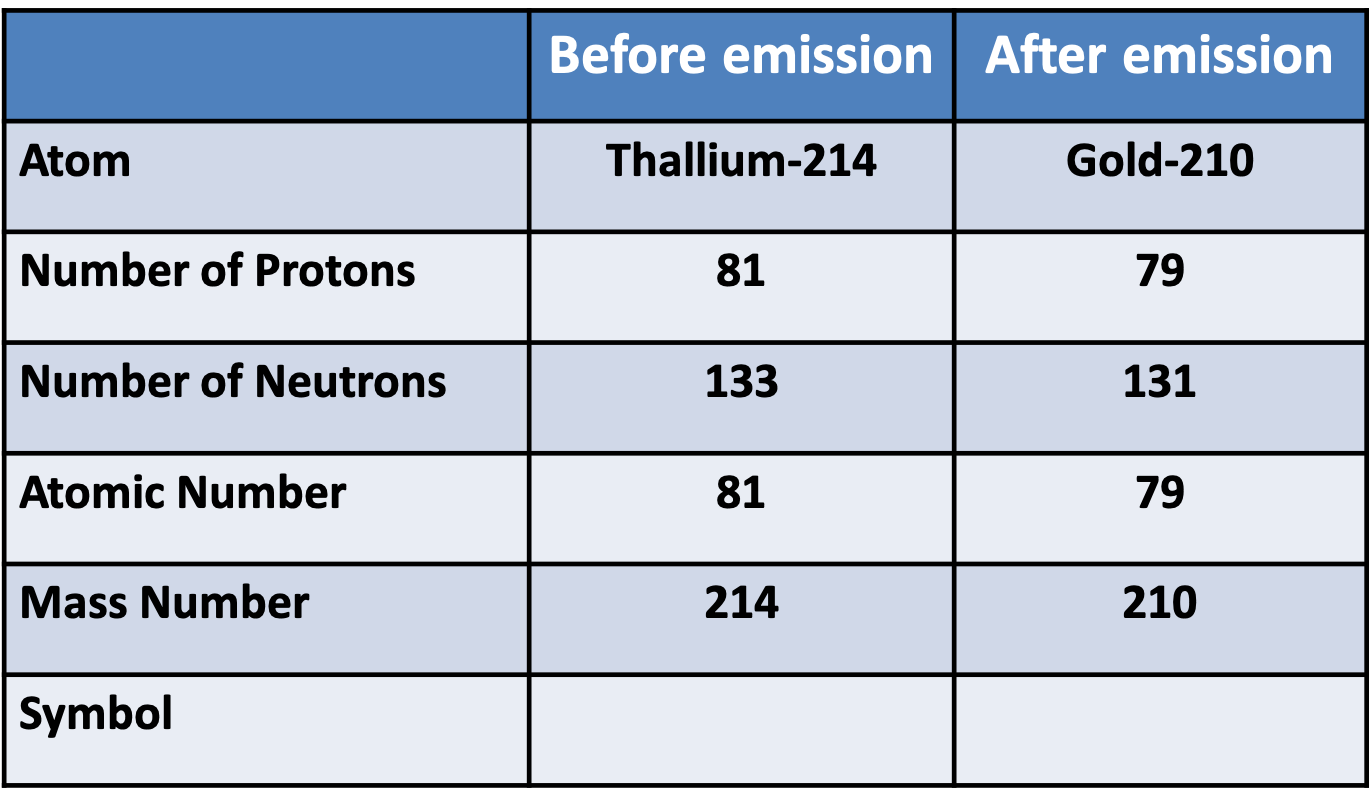
$$ begin{aligned} & {}^{214}{81}Tl ^{210}{79}Au + {}^{4}{2}He & {}^{214}{81}Tl ^{210}{79}Au + {}^{4}{2} \end{aligned}
- The mass number goes top left
- The atomic number goes bottom left
- Use an arrow like in chemistry
- You can use either \({}^{4}_{2}He\) or \({}^{4}_{2}\alpha\)
Beta Decay
Complete this cloze
In a beta emission, a neutron turns into a proton and emits a high-speed electron.
This means that the atomic number of the decay atom is ________ by ________ and the mass number _____________.
Pātai Rua: \(\beta\) Decay
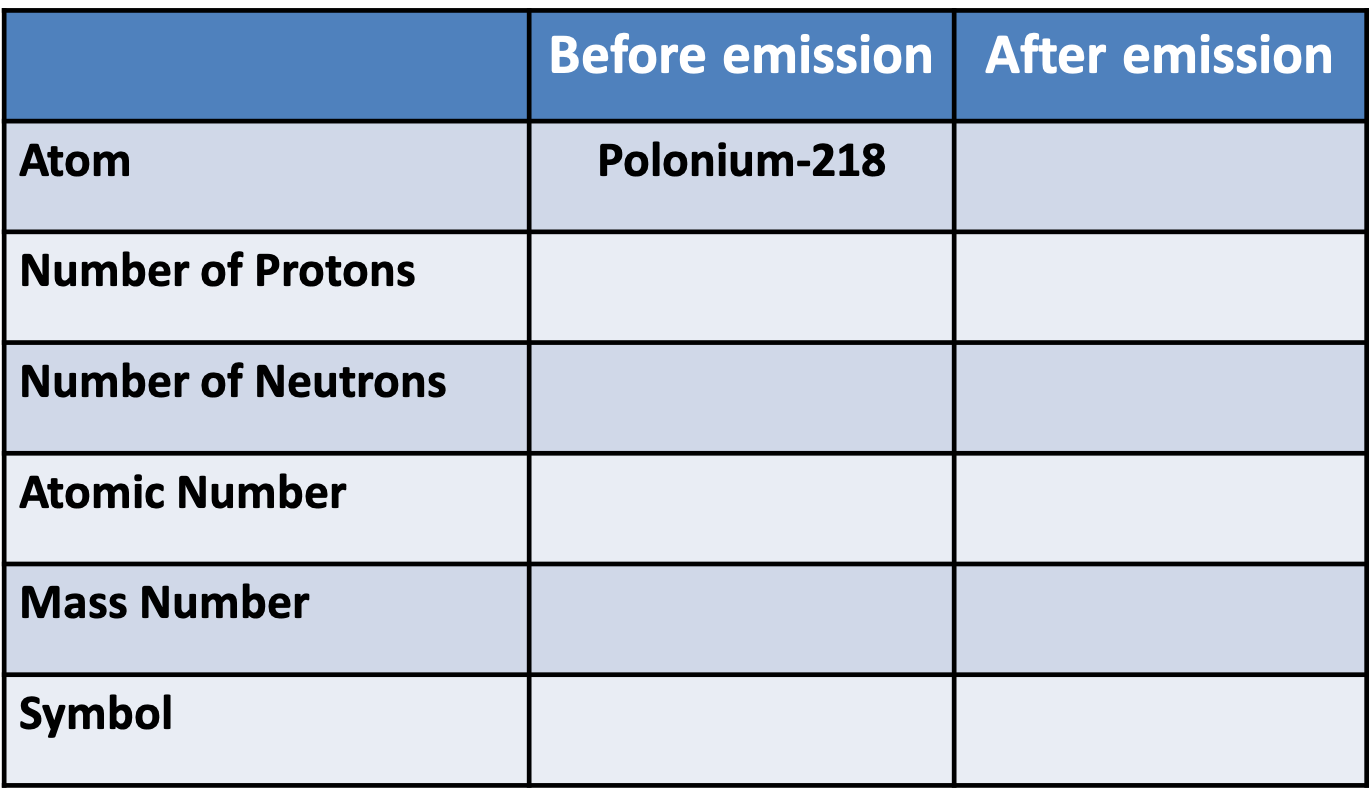
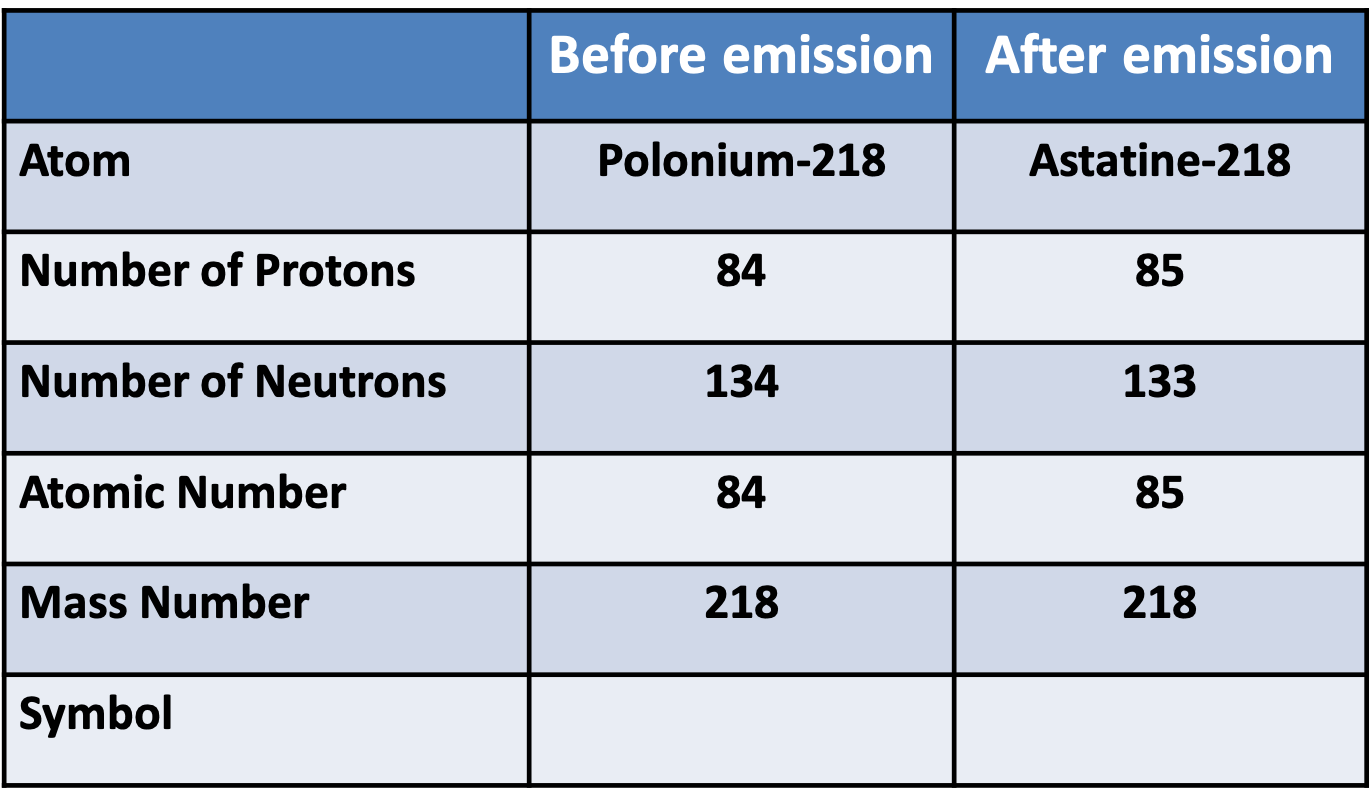
Whakatika Rua
Pātai Toru: Write a nuclear equation

Whakatika Toru
\[ begin{aligned} & {}^{218}_{84}Po \rightarrow {}^{218}_{85}At + {}^{0}_{-1}e \newline & {}^{218}_{84}Po \rightarrow {}^{218}_{85}At + {}^{0}_{-1}\beta \newline \end{aligned} \]
Gamma Decay
Complete this cloze
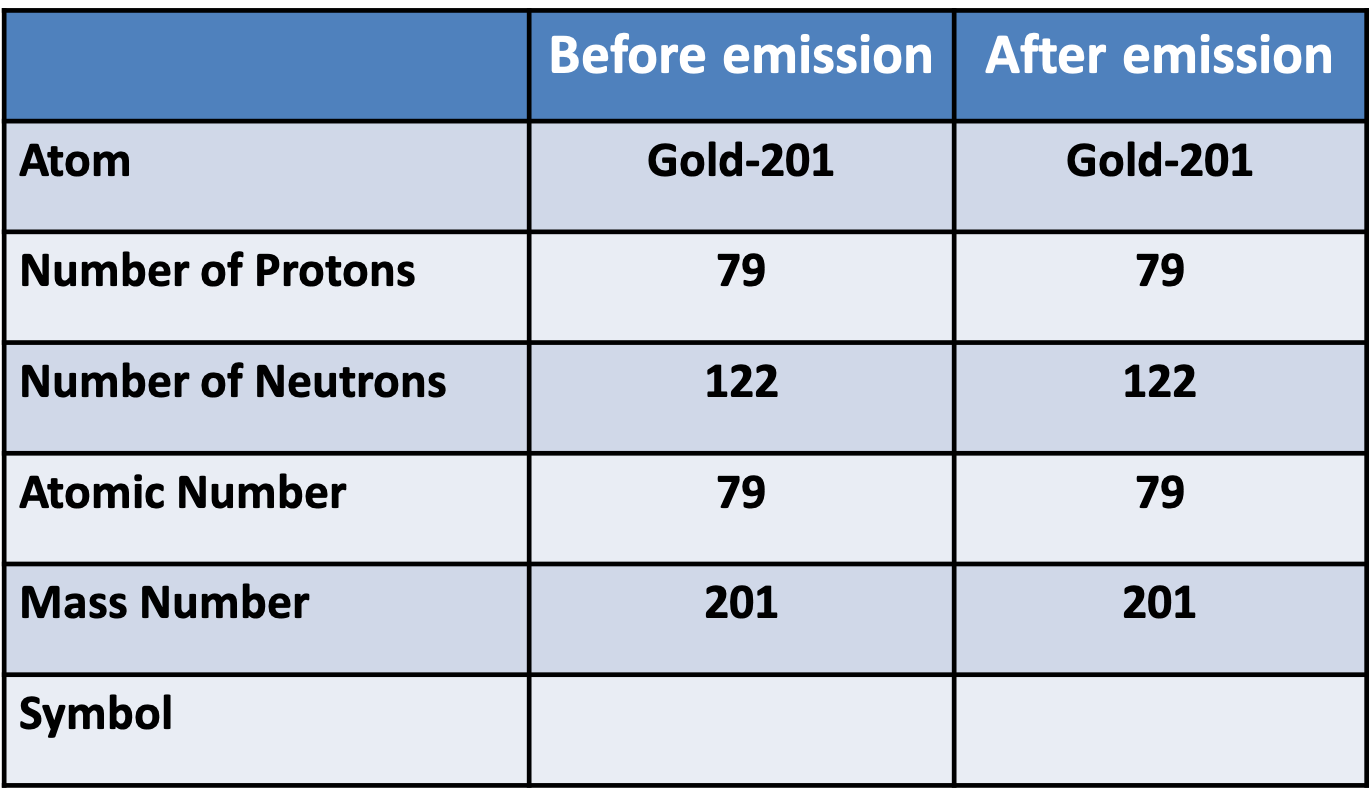
In a gamma (\(\gamma\)) emission, the decaying atom only loses ________, which means it has the ________ number of protons and neutrons afterwards.
Pātai Whā: Gamma Decay
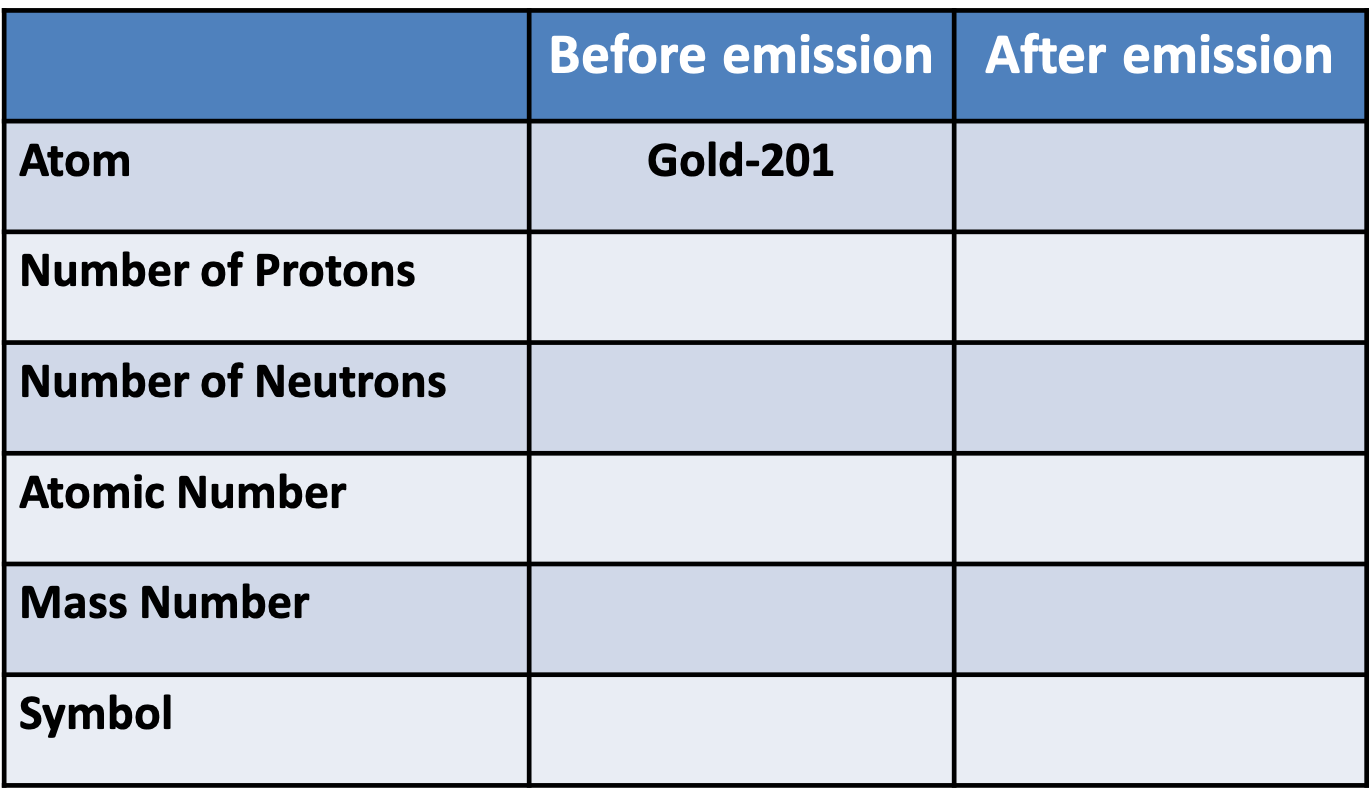
Whakatika Whā
Pātai Rimu: Write a nuclear equation

Whakatika Rimu
\[ begin{aligned} & {}^{201}_{79}Au \rightarrow {}^{201}_{79}Au + \gamma \newline \end{aligned} \]
Ngā Pātai: Write the following equations:
- An alpha decay of Polonium-218
- A beta decay of Hydrogen-3
- A gamma decay of Carbon-14
- An absorption of a neutron by Carbon-13
Ngā Whakatika 1-4
\[ begin{aligned} & {}^{218}_{84}Po \rightarrow {}^{214}_{82}Pb + {}^{4}_{2}He \newline & {}^{3}_{1}H \rightarrow {}^{3}_{2}He + {}^{0}_{-1}\beta \newline & {}^{14}_{6}C \rightarrow {}^{4}_{6}C + \gamma \newline & {}^{13}_{6}C + {}^{1}_{0}n \rightarrow {}^{14}_{6}C \end{aligned} \]
- A double alpha decay of Uranium-234
- A double alpha decay of Radon-222
- A double beta decay of Thorium-234
- A double beta decay of Bismuth-210
Ngā Whakatika 5-8
\[ begin{aligned} & {}^{234}_{92}U \rightarrow {}^{226}_{88}Ra + 2{}^{4}_{2}He \newline & {}^{222}_{88}Ra \rightarrow {}^{216}_{84}Po + 2{}^{4}_{2}He \newline & {}^{234}_{90}Th \rightarrow {}^{234}_{92}U + 2{}^{0}_{-1}\beta \newline & {}^{210}_{83}Bi \rightarrow {}^{210}_{85}At + 2{}^{0}_{-1}\beta \end{aligned} \]