Half Life
12PHY - Atomic and Nuclear Physics
Finn Le Sueur
2024
Akoranga 7 Mahi Tuatahi
- Date your book and collect a container of dice from the front
- Count the total number of dice
- Roll all the dice in one go. Set aside the dice with dots facing up, count the dice without dots and record that value.
- Roll all the dice in one go that did did not land with dots facing up. Discard those with dots facing up, count, record and repeat until no dice remain.
- Create a line graph showing the number of dice rolled (y-axis) vs the trial number (x-axis)
What you have graphed is an exponential decay curve! This is what nature does - we can observe it all over the place, even in electrical circuits.
It is not important to understand why at the moment, just to know that it does occur.
Mahi Tuatahi 2
- A Carbon-14 nucleus emits a beta particle, then the daughter nucleus also emits a beta particle immediately after. Write two equations to show this.
- An Uranium-241 nucleus emits an alpha particle AND a beta particle. Write down the equation.
- An atom of Carbon-11 absorbs a neutron. Write down the nuclear equation.
| \[ \begin{aligned} {}^{14}_{6}C \rightarrow {}^{14}_{7}N + {}^{0}_{-1}\beta \newline {}^{14}_{7}N \rightarrow {}^{14}_{8}O + {}^{0}_{-1}\beta \end{aligned} \] |
| \[ \begin{aligned} {}^{241}_{92}U \rightarrow {}^{237}_{87}Fr + {}^{4}_{2}\alpha + {}^{0}_{-1}\beta \end{aligned} \] |
| \[ \begin{aligned} {}^{11}_{6}C + {}^{0}_{1}n \rightarrow {}^{12}_{6}C \end{aligned} \] |
Ngā Whāinga Ako
- Be able to make half-life graphs
- Be able to interpret half-life graphs
Write ngā whāinga ako in your books
Half-Life
The half-life is the time taken for half of the undecayed atoms in a sample to decay.
- Radioactive materials have unique half-lives.
- It is impossible to predict when an unstable/radioactive atom will disintegrate (decay), because the actual timing of the decay is random.
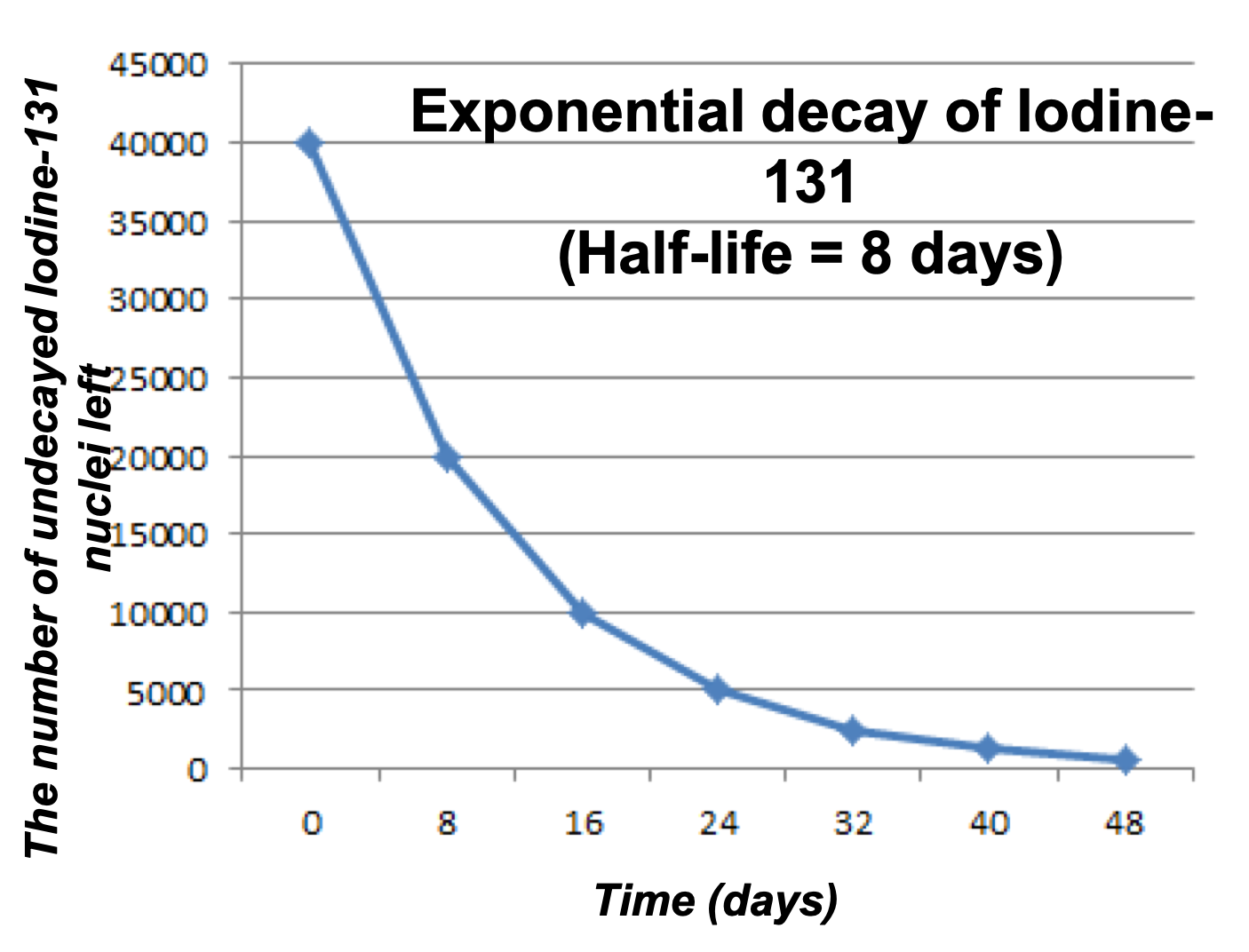
Tauira
A small sample of a radioactive material iodine-131 has been observed for several days while it decayed into xenon-131. Read the below and determine its half-life.
- On the first day, the sample contained 40,000 iodine-131 nuclei.
- Eight days later, the sample only had 20,000 iodine-131 nuclei left.
- Another eight days later, the sample had 10,000 iodine-131 nuclei left.
Iodide-131 Half-Life Graph
Pātai Tahi
The half-life of Hydrogen-3 is approximately 12.25 years. If you found a small sample of Tritium containing 5,000,000 undecayed nuclei.
- How many nuclei will be left after 12.25 years
- How many nuclei will be left after 24.5 years
- How many nuclei will be left after 49 years
- How many nuclei will be left after 196 years
- How long until there is less than 2500 undecayed nuclei left?
Whakatika Tahi
- 2,500,000
- 1,250,000
- 312,500
- 76.29
- Between 10-11 half-lives
Akoranga 8 Mahi Tuatahi
You found a \(50 g\) sample of Cobalt-60. The half-life of Cobalt-60 is 5 years. What would be the mass of the Cobalt-60 sample after 20 years?
- Estimate how long it would take for the mass of the 50 g sample to fall just below \(1.17 g\).
- Sketch a mass vs. time graph of the Cobalt-60 sample over a 30-year period.
- Use the graph to estimate the mass of the sample after 12.5 years.
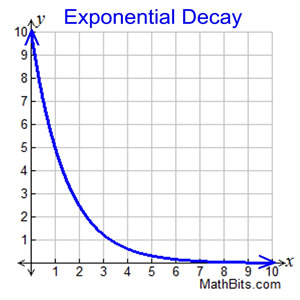
Exponential Decay Curves
- An exponential decay curve flattens out over time
- For any section, the graph is steeper on the left than the right
- This means, the mass/activity/number of atoms is changing more rapidly on left than the right
Predictions
- If the dots were joined with straight lines, the mid-point should be half way
- But, because it is an exponential curve, the midpoint of nuclei remaining/mass/activity is actually slightly less than half way
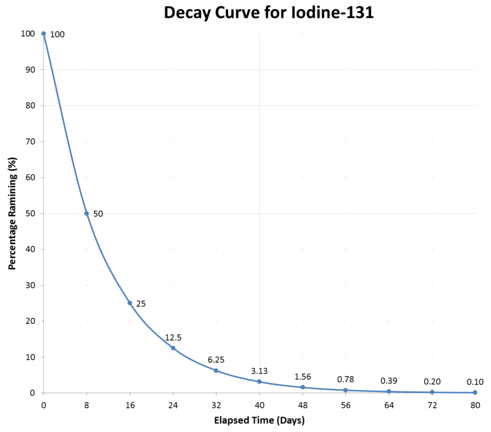
Pātai: Predict time until 37.5% left

Homework / Mahi Kāinga
- Due Monday, marked and corrected
- Half-Life 2006 Q4, Atom Models Q4, Radioactivity Q8