Writing Equations
11SCI - Chemical Reactions
Finn Le Sueur
2024
Mahi Tuatahi / Do Now
- Collect and glue in a sheet with named elements, ions and compounds.
- Elements quiz!
- If you scored 100% twice in a row, move on to the ions quiz.
When finished, get out your device and start working on your experiment document from last period
Ngā Whāinga Ako
- To write word equations to show a chemical reaction
- To write symbol equations to show a chemical reactions with symbols of state
Chemical Reactions
There are two ways we can show a chemical reaction on paper:
- Word equations
- Symbol equations
Word Equations
All equations have three parts to them:
- Reactants (left-hand side)
- Products (right-hand side)
- Arrow - means goes to form not equals
\[ \begin{aligned} reactant1 + reactant2 \rightarrow product1 + product2 \end{aligned} \]
Ngohe/Task: Write a word equation for this reaction
Ella puts 1cm of magnesium strip in 5ml of dilute hydrochloric acid and observes hydrogen gas put off and that the magnesium dissolves into magnesium chloride.
Whakatika / Answer
Ngohe/Task: Write a word equation for this reaction
Jake adds 1ml of copper sulfate to 1ml of sodium hydroxide and predicts that copper hydroxide and sodium sulfate will be produced.
Whakatika / Answer
Symbol Equations
Symbol equations are very similar, except we use the symbols from the periodic table and we have to give the state of each reactant and product. The state goes in the subscript after the element/compound.
Pātai: Discuss with your partner, what are the four states of matter that we use in chemistry?
| Solid | Liquid | Gas | Aqueous | |
|---|---|---|---|---|
| Subscript | (s) | (l) | (g) | (aq) |
- Aqueous means that it is dissolved in solution
- (l) is very uncommon in Year 11, (aq) is much more common
Ngohe/Task: Write a symbol equation for this reaction
Ella puts 1cm of magnesium strip in 5ml of dilute hydrochloric acid and observes hydrogen gas put off and that the magnesium dissolves into magnesium chloride.
\[ \begin{aligned} Mg_{(s)} + HCl_{(aq)} \rightarrow H_{2(g)} + MgCl_{(aq)} \end{aligned} \]
Ngohe/Task: Write a word and symbol equation for this reaction
Jake adds 1ml of copper sulfate to 1ml of sodium hydroxide and predicts that copper hydroxide and sodium sulfate will be produced.
\[ \begin{aligned} CuSO_{4(aq)} + NaOH_{(aq)} \rightarrow Cu(OH)_{2(s)} + Na_{2}SO_{4(s)} \end{aligned} \]
Balancing Equations
- Equations are balanced by putting whole numbers in front of compounds/molecules
- We want there to be the same number of each atom on each side of the \(\rightarrow\)
- This is usually done through trial and error
Tauria / Example
\[ \begin{aligned} Magnesium Metal + Oxygen Gas &\rightarrow Magnesium Oxide \newline Mg_{(s)} + O_{2(g)} &\rightarrow MgO_{(s)} \end{aligned} \]
| RHS | LHS | |
|---|---|---|
| Mg | ||
| O |
It is unbalanced!
| RHS | LHS | |
|---|---|---|
| Mg | 1 | 1 |
| O | 2 | 1 |
Step 1
Start by balancing the oxygen by putting a two in front of the \(MgO\).
\[ \begin{aligned} Mg_{(s)} + O_{2(g)} &\rightarrow 2MgO_{(s)} \end{aligned} \]
| RHS | LHS | |
|---|---|---|
| Mg | 1 | 2 |
| O | 2 | 2 |
Step 2
Now the magnesium is unbalanced, so we need to put a two in front of the \(Mg\).
\[ \begin{aligned} 2Mg_{(s)} + O_{2(g)} &\rightarrow 2MgO_{(s)} \end{aligned} \]
| RHS | LHS | |
|---|---|---|
| Mg | 2 | 2 |
| O | 2 | 2 |
Balanced!
Summary of Symbols
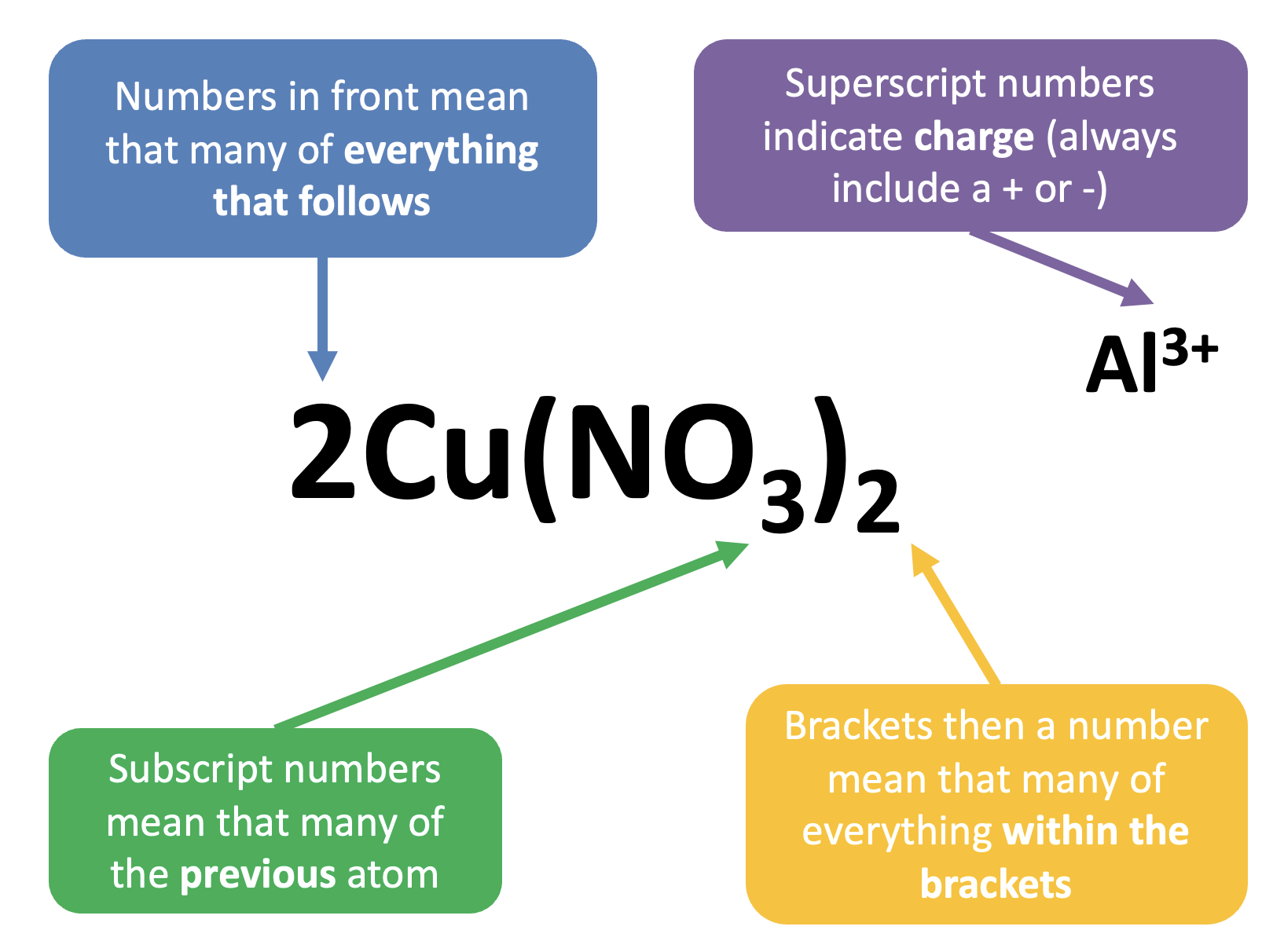
Mahi / Work
- Worksheet (work together to make sure you get it right!)
- sciPad page 30, 31 and 29.