Review of the Atom
11SCI - Chemical Reactions
Finn Le Sueur
2024
About The Unit
- Chemical Reactions
- External
- 4 Credits
Ngā Whāinga Ako
- Review the structure of the atom
Copy this diagram into your book.
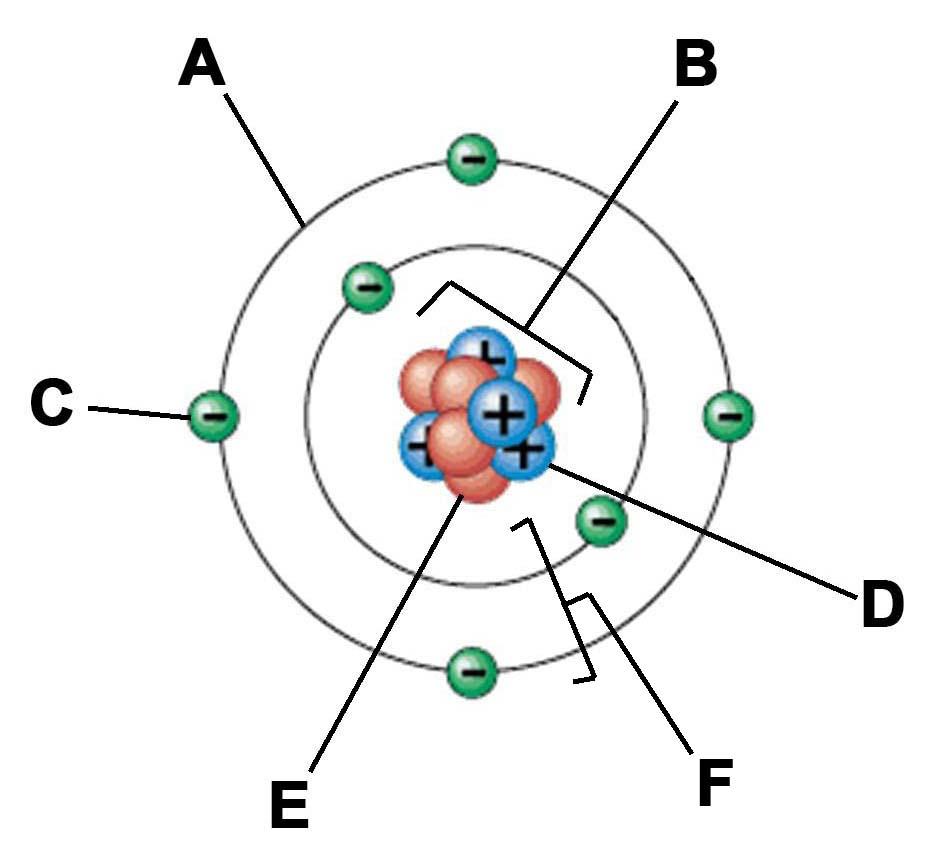
Overview
An atom is made up of two distinct parts. The nucleus and electrons.
- The nucleus is the centre of the atom (B).
- The electrons (C) move around the nucleus in a shell (A)
- If one orbit is a shell, all the shells together are called the electron shells (F)
- The nucleus holds the vast majority of the mass and is made of two particles
- The proton (D) and the neutron (E)
Atomic Number
The number of protons inside the nucleus.
Mass Number
The number of protons + neutrons inside the nucleus.
NB: In a neutral atom, the number of protons is equal to the number of electrons. Why?