Rate of Reaction
11SCI - Chemical Tūhura
Finn Le Sueur
2024
The Rate of Reaction
Understanding the rate of reaction is very important in Pūtaiao we need to be able to control it when performing reactions on an industrial scale.
The rate at which products are produced.
OR
The rate at which reactants are used up.
Here are two ways we can measure the rate of reaction:
- measuring the volume of products produced,
- measuring the weight of reactants lost
Can you think of any others?
Displacement of Gas
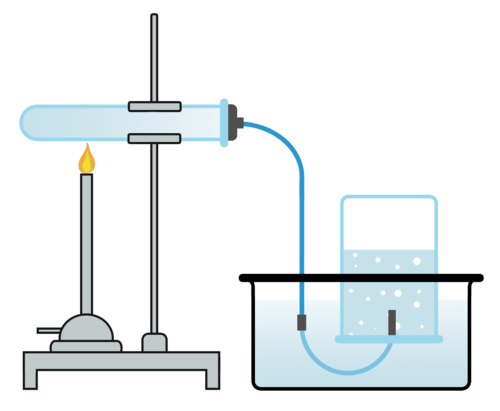
Measuring the mililitres (mls) of gas produced over a period of time.
Whakamātau: Measuring the Rate of Reaction
Method is on Google Classroom
Production of Gas Method
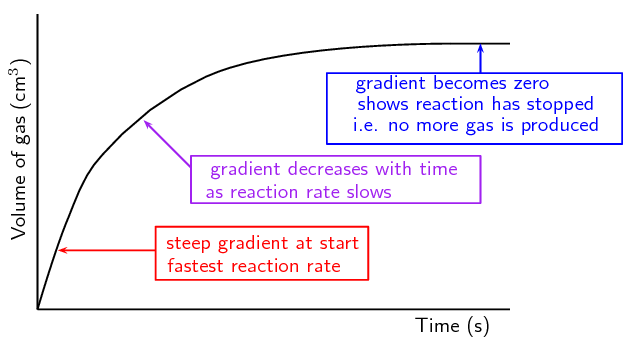
When measuring the amount of gas produced, the volume goes up over time so the graph should look like this
Loss of Mass Method
When measuring the amount of mass lost, the mass goes down so the graph should look like this.

Things to Note
- Sometimes a reaction will take a few seconds to start, this can be due to a protective coating on some metals.
- You need 4-5 iterations of your dependent variable (concentration, surface area, temperature) to draw a valid conclusion. Each iteration should be repeated at least three times so an average can be calculated.
Loss of Mass Whakamātau
Method is on Google Classroom - please open it up, read it and come to the demo bench!