Collision Theory
11SCI - Chemical Tūhura
Finn Le Sueur
2024
- AS90930
- 4 Credits
- Internal
- Experiment & written assessment
- 5 Weeks
- 3 weeks learning
- 1 week practice assessment
- 1 week real assessment
Ngā Whainga Ako:
- To explain how reactions happen due to collisions between particles,
- To explain the term activation energy,
- To state the factors that affect the rate of reaction.
Reactions, Particles and Collisions
- Particles in liquids and gases can move freely which allows them to react easily.
- A reaction can take place when particles collide.
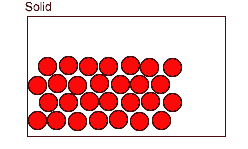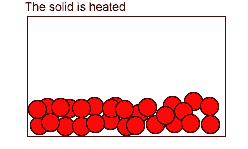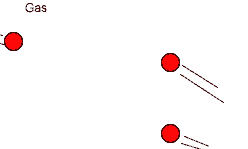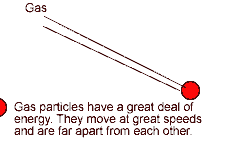
The number of reactions that occur depends on:
- the frequency of successful collisions (with correct orientation) between particles,
- and the energy with which particles collide.
If particles collide with less energy than the activation energy, they will not react. The particles will just bounce off each other.
Similarly, if they collide with the wrong orientation they will not react.
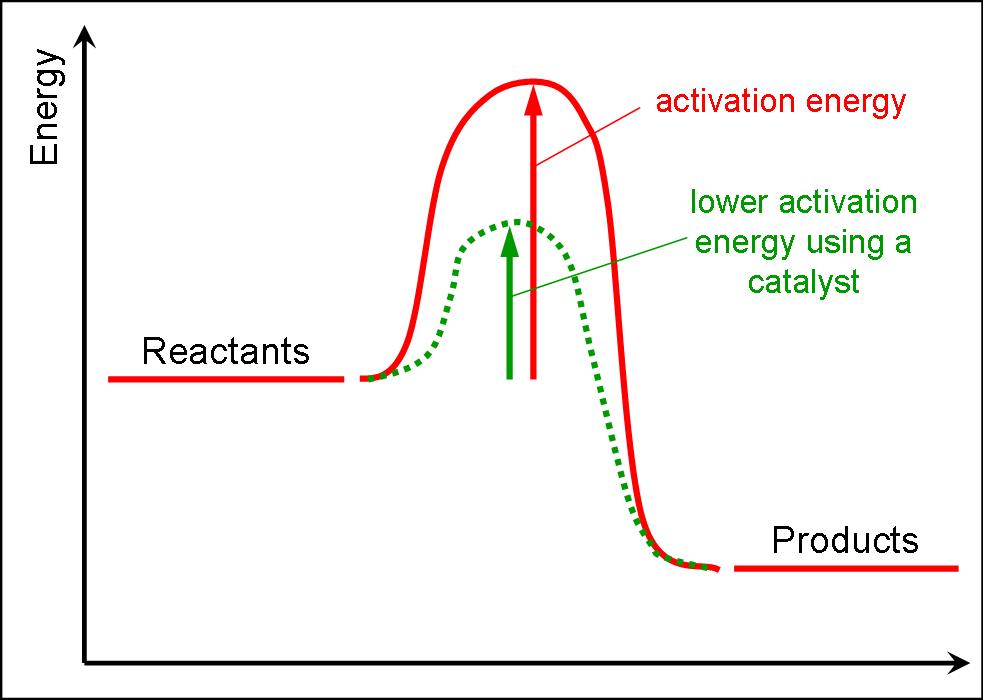
Activation Energy
The kinetic energies of the two colliding particles are added. If the sum of their energy is greater than the activation energy, the collision is successful and a reaction takes place.

Ngohe / Task
- Open Google Classroom.
- Find “Ngohe/Task: How to Get a Date?”
- Watch the video and answer the associated questions.
Ngohe / Task
- Open Education Perfect.
- Find the “Collision Theory” task.
- Work slowly through the task. Take notes in your book on the following:
- A definition of reaction that includes bonds.
- A definition and diagram of activation energy
- An analogy of activation energy
- Write the drag-and-drop sentences into your book as summary notes
- Two graphs showing products/reactants during a reaction and an explanation of the graph
- A definition of a catalyst
- Write the rates of reaction drag-and-drop sentences into your book