Changing Temperature
11SCI - Chemical Tūhura
Finn Le Sueur
2024
Ngā Whāinga Ako
- To be able to describe what a change in temperature means
- To be able to describe the collision theory impacts of temperature
- To be able to carry out a change of temperature reaction
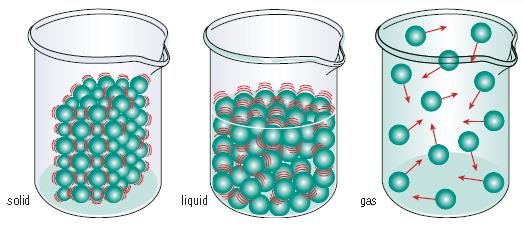
Particle Theory
- This theory gives us the model of matter as a series of atoms with energy
- The greater the energy they posses, the more they vibrate and move around
- The lowest energy particles tend to be solids (not moving, but vibrating)
- The middle are liquids (freely moving past each other)
- The highest are gasses (filling the volume of a container)
Energy
- To give matter energy, we heat it up
- This heat energy is seen as kinetic energy for individual particles (moving energy)
- As the temperature increases, they move faster
Impact on Collision Theory
- If particles are moving faster, there are more collisions occuring
- If particles are moving faster, they have more kinetic energy and are more likely to surpass the activation energy
- This means that the probability that an individual collision is successful/effective also increases
- Therefore, temperature affects both the number of collisions and the probability that they are successful (reaction occurs).
