Changing Concentration
11SCI - Chemical Tūhura
Finn Le Sueur
2024
Concentration: Collision Theory
- Concentration is easily thought of like raro - the more raro particles you add to your drink, the stronger it is!
- The more particles there are, the higher the chance there is of particles bumping into each other
- The more bumps there are, the greater number of reactions
- It does not increase the probability of successful collisions
Tauria / Example
Say 10% of collisions result in a successful reaction between two particles.
If 1000 collisions happen, there should be \(1000 \times 0.1 = 100\) successful reactions
Since concentration doesn’t change the probability (10%), it only changes the number of collisions. \(5000 \times 0.1 = 500\) successful reactions.
Matapaki / Discussion
Why does changing the concentration not change the probability of a successful reaction?
Think of the two things required for a reaction to occur!
Whakatika
Changing temperature does not affect the probability of a successful reaction because it does not change:
- the activation energy,
- the kinetic energy of the particles (temperature/speed),
- or the likelihood that they collide in the correct orientation.
Dilutions
- To change the concentration we must dilute one of our reactants.
- Pātai: What do we dilute raro with?
- Whakatika: Water! \(H_{2}O\)
- Pātai: Why do we dilute only one reactant?
- Whakatika: To ensure we are changing only one thing in our experiment so it is a fair test!
Dilutions Explained
- In Year 11 we will do dilutions in percentages. For example, 90% \(HCl\), 50% \(HCl\) or 30% \(HCl\)
- For \(10ml\) of 80% \(HCl\) this means 80% of the \(10ml\) is \(HCl\) and 20% is water!
\(\text{Volume} = \frac{percentage}{100} \times \text{total volume}\)
Pātai: Calculate the volumes of each solution
\(\text{Volume of Acid} = \frac{percentage}{100} \times \text{total volume}\)
| Concentration | Volume | \(ml\) of \(HCl\) | \(ml\) of \(H_{2}O\) |
|---|---|---|---|
| 90% | 100ml | ||
| 80% | 75ml | ||
| 75% | 50ml | ||
| 50% | 50ml | ||
| 30% | 50ml |
| Concentration | Volume | \(ml\) of \(HCl\) | \(ml\) of \(H_{2}O\) |
|---|---|---|---|
| 90% | 100ml | \(90ml\) | \(10ml\) |
| 80% | 75ml | ||
| 75% | 50ml | ||
| 50% | 50ml | ||
| 30% | 50ml |
| Concentration | Volume | \(ml\) of \(HCl\) | \(ml\) of \(H_{2}O\) |
|---|---|---|---|
| 90% | 100ml | \(90ml\) | \(10ml\) |
| 80% | 75ml | \(60ml\) | \(15ml\) |
| 75% | 50ml | ||
| 50% | 50ml | ||
| 30% | 50ml |
| Concentration | Volume | \(ml\) of \(HCl\) | \(ml\) of \(H_{2}O\) |
|---|---|---|---|
| 90% | 100ml | \(90ml\) | \(10ml\) |
| 80% | 75ml | \(60ml\) | \(15ml\) |
| 75% | 50ml | \(37.5ml\) | \(12.5ml\) |
| 50% | 50ml | ||
| 30% | 50ml |
| Concentration | Volume | \(ml\) of \(HCl\) | \(ml\) of \(H_{2}O\) |
|---|---|---|---|
| 90% | 100ml | \(90ml\) | \(10ml\) |
| 80% | 75ml | \(60ml\) | \(15ml\) |
| 75% | 50ml | \(37.5ml\) | \(12.5ml\) |
| 50% | 50ml | \(25ml\) | \(25ml\) |
| 30% | 50ml |
| Concentration | Volume | \(ml\) of \(HCl\) | \(ml\) of \(H_{2}O\) |
|---|---|---|---|
| 90% | 100ml | \(90ml\) | \(10ml\) |
| 80% | 75ml | \(60ml\) | \(15ml\) |
| 75% | 50ml | \(37.5ml\) | \(12.5ml\) |
| 50% | 50ml | \(25ml\) | \(25ml\) |
| 30% | 50ml | \(15ml\) | \(35ml\) |
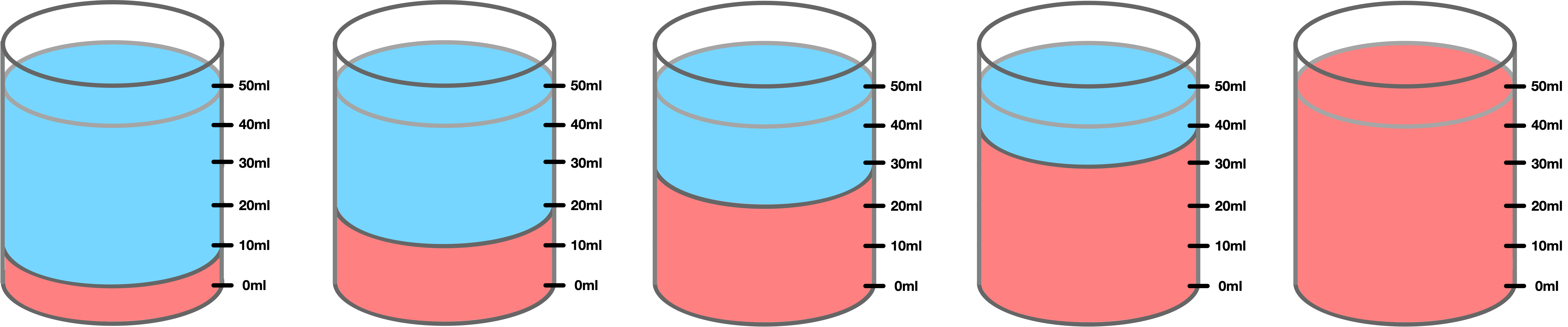
Diagrams
Glue in the diagram and label each beaker with a concentration percentage. Red is acid and blue is water.