Electron Configurations
pHun Reactions - 10SCIE
Finn Le Sueur
2024
Akoranga Mahi Tuatahi
You may spend 15 minutes working on cutting out, and filling in your flashcards on the first 20 elements!
If you finish that, you may spend the remainder of the time practising with the flashcards 😃
Te Whāinga Ako
- Write electron configurations for the first 20 elements
Write the date and te whāinga ako in your book
Recap: Electrons

- Negatively charged
- Very small
- Light
- Move fast
- Exist in orbitals/shells around the nucleus of atoms
- In a neutral atom, there are the same number of electrons as protons
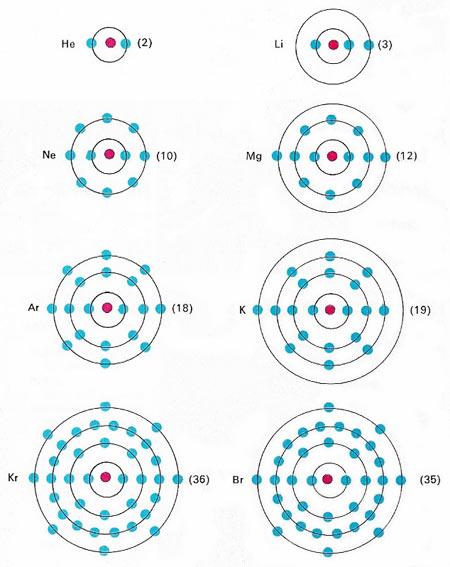
Electron Arrangements
- Electrons are behind all chemical reactions
- They can be taken from one atom/molecule and given to another one - thus causing a chemical reaction!
- They go around an atom in shells, but how many go in each shell?
- Fill the shells from the inside to the outside!
- Draw electrons as crosses: x
- First Shell
- Can hold up to 2 electrons
- Second Shell
- Can hold up to 8 electrons
- Third Shell
- Can hold up to 18 electrons
Pātai: Magnesium
- What is the symbol for magnesium?
- How many protons does magnesium have?
- How many electrons does magnesium have?
- Write the electron configuration, e.g.: 2, 3
- Draw the electron configuration diagram (remember to use crosses!)
Ngohe/Task
- Find your worksheet from last class
- For each element, write the electron configuration:
- Hint: See the Carbon row!
- Collect an electron configuration worksheet from
the front and draw the diagrams!
- Hint: Draw electrons as crosses like so: x
Tākaro/Game
- Form into pairs
- Send one person to collect two battleship boards
- Follow the instructions on the front to play the game!