Akoranga 5 Mahi Tuatahi 🔗
- 10 min: Finish making or practise your flashcards
- 10 min: Complete the electron configuration worksheet
Ngā Whāinga Ako 🔗
- Can describe why ions form and identify the difference between cations & anions
- Locate groups 1, 2, 16, 17, 18 elements on the periodic table and identify their number of valence electrons
- Describe how ionic bonds are formed from the transfer of electrons
Write the date and ngā whāinga ako in your book
Ions 🔗
An atom or molecule that has gained or lost electrons, and has become charged.
- Atoms are more stable when they have a full valence (outer) shell
- Atoms with an almost full valence shell will take electrons from other atoms
- Atoms with not many valence electrons will donate electrons to other atoms

Tauria/Example: Sodium Fluoride 🔗

- Sodium only has one valence electron, and can donate it
- Fluorine is only one electron away from a full shell, so will take one
- Sodium becomes positively charged because it lost an electron
- Fluorine becomes negatively charged because it gained an electron
- They are attracted together and form sodium fluoride
- This is called an ionic bond - a bond between ions
Tauria/Example: Sodium Chloride 🔗
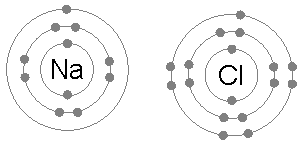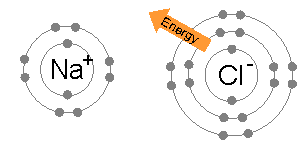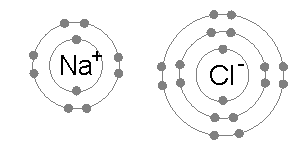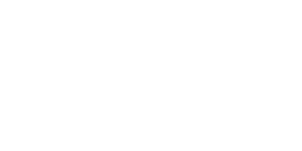
- Sodium only has one valence electron, and can donate it
- Chlorine is only one electron away from a full shell, so will take one
- Sodium becomes positively charged because it lost an electron
- Chlorine becomes negatively charged because it gained an electron
- They are attracted together and form sodium chloride
Which Atoms Form Ions? 🔗
- Only atoms in groups 1, 2, 16, and 17 ions
- Groups are vertical columns on the periodic table
Open your periodic table an find groups 1, 2, 16 and 17
Highlight these columns and add a label saying they will form ions.
- Open to your ionic configuration diagrams
- Look at the valence shell of groups 1, 2, 16, 17 and 18
- Pātai: What do all the elements in each group have in common?
- Elements in group 1 have 1 valence electron
- Elements in group 2 have 2 valence electrons
- Elements in group 18 have a full valence shell
- Elements in group 17 are missing 1 electron
- Elements in group 16 are missing 2 electrons
What is Special About Group 18? 🔗
- They have a full valence shell
- This means they do not want to take or give any electrons
- This means they are already very stable
- This means they do not react!
Akoranga 7 Mahi Tuatahi 🔗
Work in pairs to answer these questions:
- What is the formula of the hydrogen ion?
- What is the formula of the oxide ion?
- What is the formula of the iron (II) ion?
- What is the name of the $OH^{-}$ ion?
- What is the name of the $Cl^{-}$ ion?
Ngā Whāinga Ako 🔗
- Be able to define cation and anion
- Be able to name ionic compounds
Write the date and ngā whāinga ako in your book
Cations 🔗
Cation: An atom that has lost electrons to become positively charged
- Pātai Tahi: Write three examples of cations using your knowledge of ions
- Pātai Rua: Use your periodic table to determine what type of elements form cations
Anions 🔗
Anion: An atom that has gained electrons to become negatively charged
- Pātai Tahi: Write three examples of anions using your knowledge of ions
- Pātai Rua: Use your periodic table to determine what type of elements form anions