Products of Combustion
10SCIE - Fire & Fuels
Finn Le Sueur
2024
Akoranga 6 Mahi Tuatahi
- Collect a crossword as you come in
- Glue it into your book
- You have 10min to complete the crossword!
Ngā Whāinga Ako
- Be able to describe the products of combustion
- Be able to perform the pop, limewater and lighted splint gas tests
- Be able to determine the products of combustion through experiment
Write the date and ngā whāinga ako in your book.
Combustion
- Combustion is a very predictable formula. Every time something is burned, water is produced, along with a mixture of carbon dixoide, carbon monoxide and carbon.
- It can be a chain reaction
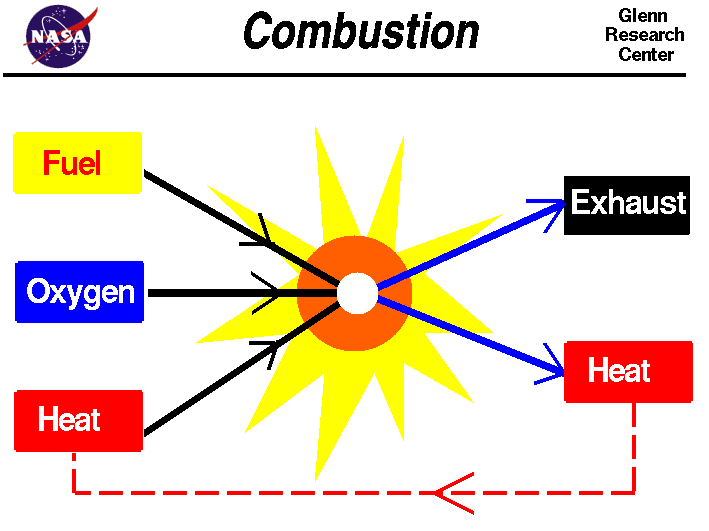
Collect a products of combustion sheet and glue it into your exercise book!
Testing for Water

- In fact we can use cobalt (II) chloride
strips
- The paper goes pink in the presence of water
- It can be dehydrated and re-used
Fill out the water test section of your products of combustion sheet!
Rangahau/Research
- In pairs, try and recall from Y9, the tests for carbon dioxide, oxygen and hydrogen gas.
- Get out a device and research the gas tests, just to be sure.
- Add notes and diagrams to your Products of Combustion sheet.
Whakamātau
- Open to page 7 of your workbook: What is produced when a candle burns?
- Read through the whakamātau carefully
- Come up to the demo bench
- Form into groups of 3-5 and collect the required equipment
- Conduct the whakamātau (20min)
- Clean up your station